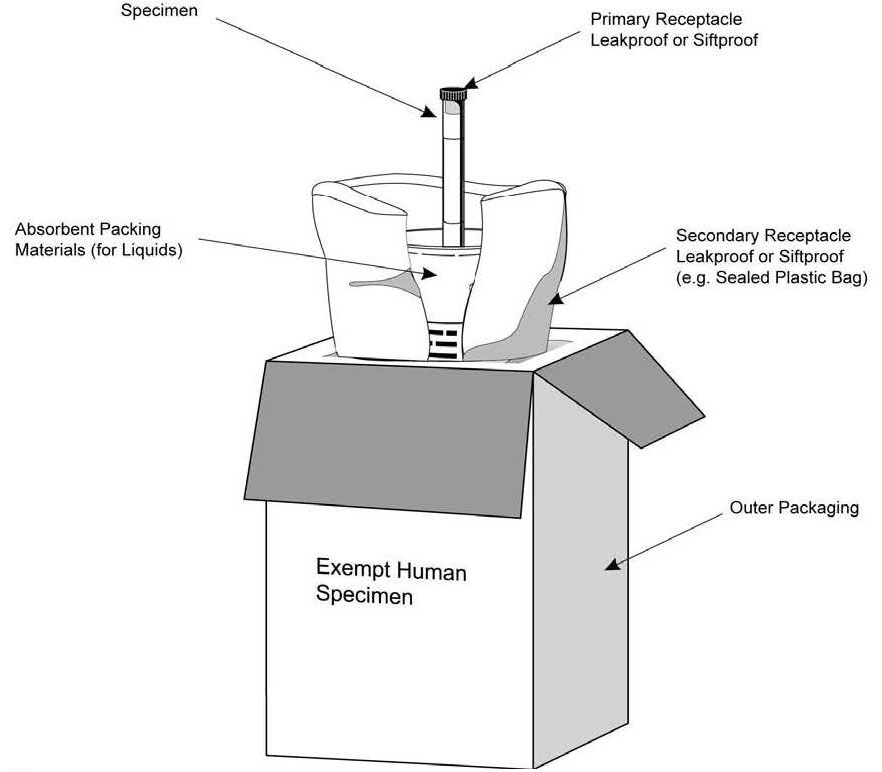
42 examples of exempt human specimens
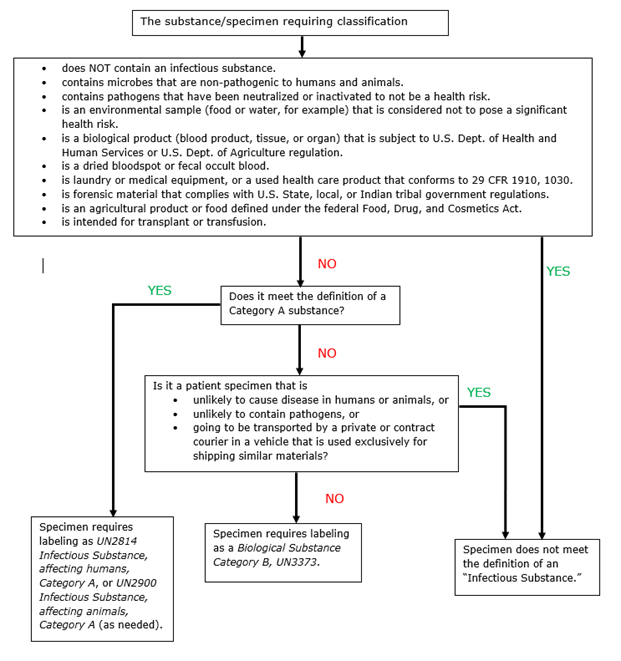
Exemptions (2018 Requirements) | HHS.gov §46.104 Exempt research. (a) Unless otherwise required by law or by department or agency heads, research activities in which the only involvement of human subjects will be in one or more of the categories in paragraph of this section are exempt from the requirements of this policy, except that such activities must comply with the requirements of this section and as specified in each category. Shipping infectious substances - Transport Canada Patient specimens are those collected directly from humans or animals and include, for example, excreta, blood and its components, tissue and tissue fluids swabs, and body parts being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention. Cultures versus patient specimens
Exempt Animal or Human Specimens | Environment, Health and Safety "Exempt Animal Specimen" The package must consist of three components: Leek-proof primary receptacle Leak-proof secondary packaging For liquids, absorbent material sufficient to absorb the entire contents must place between the primary receptacle and the secondary packaging
Examples of exempt human specimens
Definition of Human Subjects Research | grants.nih.gov It summarizes Exemptions 1, 2, 3, 4, 5, 6, 7 and 8, providing basic definitions, examples of studies that meet and do not meet the criteria of the exemption, and aspects one must consider when engaged in exempt or non-exempt human subjects research. Research Involving Private Information or Biospecimens Flowchart IATA Dangerous Goods Regulations | IATA Requirements | Therapak Examples of those tests are: blood or urine for cholesterol levels, hormone levels, prostate specific antigens (PSA), tests to monitor organ function (heart, liver, kidney), tests conducted for insurance or employment, pregnancy, biopsies for cancer, drug or alcohol presence testing. PDF 1 Meets the definition of human subjects research. Meets the definition of human subjects research. Exempt studies involve human subjects research: research involving a living individual about whom data ... specimens if publicly available, or recorded such that subjects ... these are possible examples only. Final determination of exemptions should be made in accordance with 45 CFR 46.
Examples of exempt human specimens. Category B - UN3373.com Examples of specimens which may be transported under this paragraph include the blood or urine tests to monitor cholesterol levels, blood glucose levels, hormone levels, or prostate specific antibodies (PSA); those required to monitor organ function such as heart, liver or kidney function for humans or animals with non-infectious diseases, or for … Shipping Biological Substances FAQs | UPS - United States What is an Exempt Human Specimen or Exempt Animal Specimen? "Patient specimens for which there is minimal likelihood that pathogens are present." (Dangerous Goods Regulations, 3.6.2.2.3.8) Refer to 49 USC § 173.134 for additional information regarding domestic shipping. PDF Step 3: Packing Category A and B and Exempt Human and Exempt Animal ... Step 3: Packing Category A and B and Exempt Human and Exempt Animal Specimens Job Aid . Use the pages below as a reference for packing Category A, B, and Exempt Specimens. Category A Substance Packaging . NOTE: The packaging is the same for both types (UN 2814 and UN2900) of Category A packaging, only the UN mark and Proper Shipping Names change. How to Ship Clinical Samples | FedEx For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.
Exemption Categories | Research at Brown | Brown University Examples: Benign behavioral interventions could include having participants play an online game, having them solve puzzles under various environmental conditions, or having them decide how to allocate a nominal amount of cash between themselves and others. Research Using Human Biological Specimens Specifically, exempt category #4 applies to research that involves the collection or study of existing * data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available ** or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or … Guidelines for the Transportation of Clinical Human Samples Staff may use private vehicles to transport exempt human samples They must adhere to the following criteria: - These individuals must be adequately trained and made aware of the potential hazards contained within the packages (e.g Bloodborne Pathogen Awareness training). PDF 1 Meets the definition of human subjects research. Meets the definition of human subjects research. Exempt studies involve human subjects research: research involving a living individual about whom data ... specimens if publicly available, or recorded such that subjects ... these are possible examples only. Final determination of exemptions should be made in accordance with 45 CFR 46.
IATA Dangerous Goods Regulations | IATA Requirements | Therapak Examples of those tests are: blood or urine for cholesterol levels, hormone levels, prostate specific antigens (PSA), tests to monitor organ function (heart, liver, kidney), tests conducted for insurance or employment, pregnancy, biopsies for cancer, drug or alcohol presence testing. Definition of Human Subjects Research | grants.nih.gov It summarizes Exemptions 1, 2, 3, 4, 5, 6, 7 and 8, providing basic definitions, examples of studies that meet and do not meet the criteria of the exemption, and aspects one must consider when engaged in exempt or non-exempt human subjects research. Research Involving Private Information or Biospecimens Flowchart

.png?width=1200&height=627&ext=.png)










Komentar
Posting Komentar