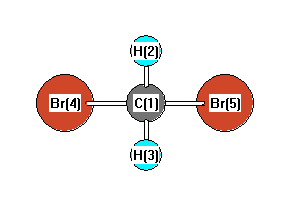
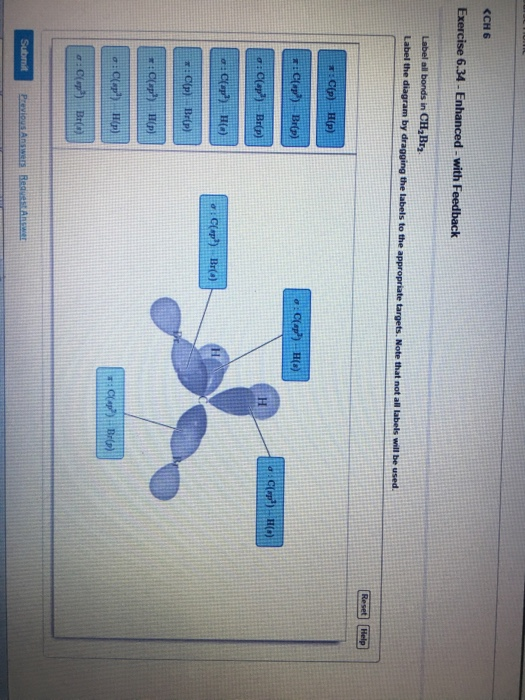
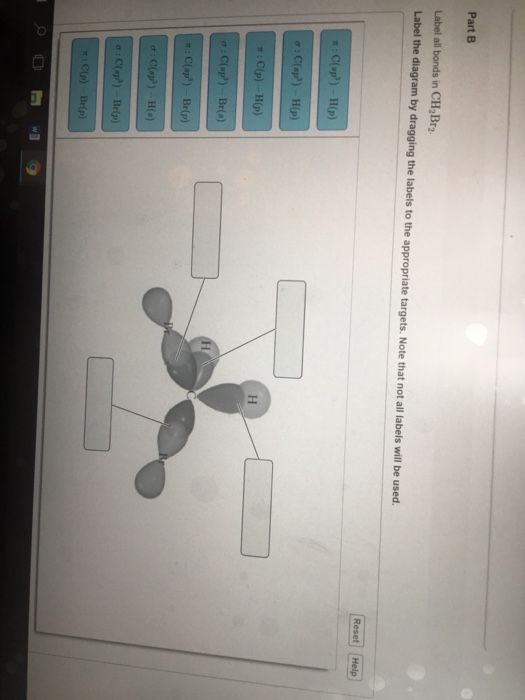
42 label all bonds in ch2br2.
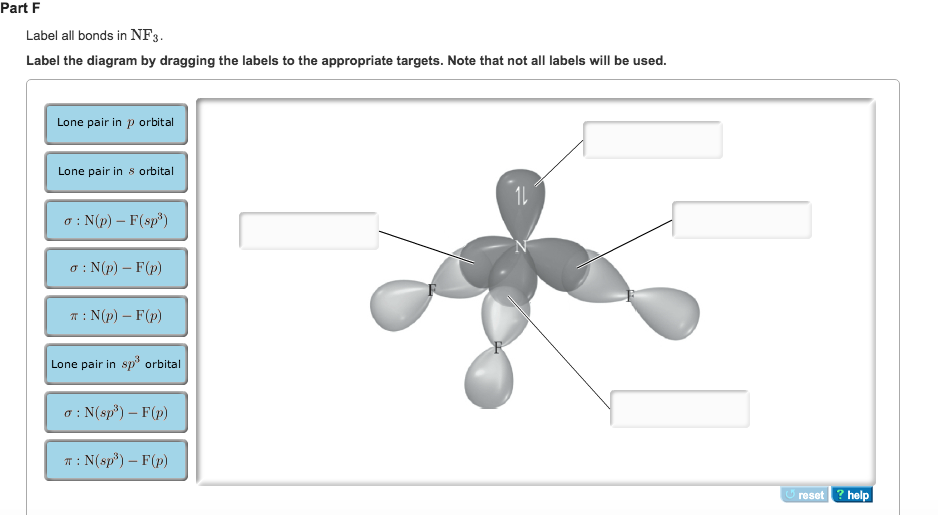
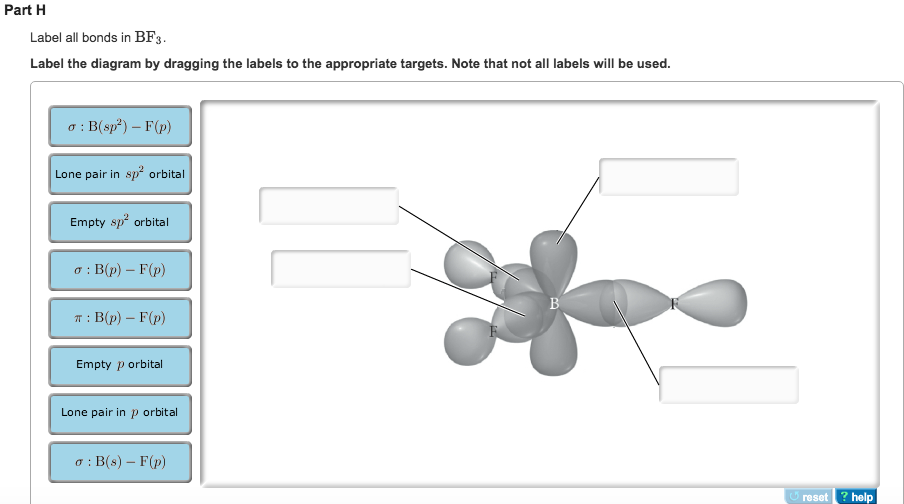
Chapter 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... VSEPR Theory. Combines the Lewis Model with the idea that valence electron groups repel one another (Covalent Bonds) to predict the general shape of a molecule from its Lewis Structure. Repulsions between electron groups on the interior atoms of a molecule determine the geometry of the molecule, and the preferred geometry is the one in which ... Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... The fluorides BF3, AlFz, SiF4, and PFs are Lewis acids. The all form very stable fluoroanions when treated with lithium fluoride. In contrast, the three fluorides CF4, NF3, and SF do not react with lithium fluoride. Explain by selecting all that apply. O CFA and NF, have no available orbitals on the inner atoms to form additional bonds.
How many bonds are in CH2Br2? - Answers Does CH2Br2 form hydrogen bonds? No, hydrogen bonding only occurs in compounds where hydrogen (H) is bonded to nitrogen (N), oxygen (O) or fluorine (F). How many isomers of ch2br2? This compound...

Label all bonds in ch2br2.
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B (p) - F (p) Empty p orbital Lone pair in p orbital B B (sp²) - F (p) в : В (8) — F (p) o : B (p) - F (p) Empty sp ... Answered: In the sketch of the structure of… | bartleby Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Label each molecule with the name of its shape. - Brainly.com The shape is mostly the one that results in the least electronic repulsions. The table of the molecular shape that corresponds to the different number of electron pairs is attached in the image. In , silicon has four valence electrons that are utilized in the formation of four Si-F bonds. Such four bonds are best stabilized in the tetrahedral ...
Label all bonds in ch2br2.. Label all bonds in CH2Br2. Label all bonds in SO2 ... - Trustsu The following solution is suggested to handle the subject “Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in NF3. Label… CH 6 Label all bonds in CH2Bry Reset Help C(P) HF) label all bonds in CH2Br2- Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. N2H2 (skeletal structure HNNH) b. Chemistry: Semester 2, Unit 1 Practice Problems - Quizlet 65: Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7. a. N2H2 (skeletal structure HNNH)
Use valence bond theory to write the hybridization and ... - Socratic Warning! Long Answer. Here's what I get. > Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two "C" atoms (least electronegative) will be the central atoms, with the "N" attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula "NCCH"_3 tells you that the three "H" atoms are attached to the terminal carbon atom. Solved Label all bonds in CH2Br2. ... - Chegg Best Answer 92% (63 ratings) Transcribed image text: Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Previous question Next question Solved Part B Label all bonds in CH2Br2 ... - Chegg Transcribed image text: Part B Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used H (p) H (p) Br (o) Br (p) Previous question Next question Ch2br2 Identify The Of Atom The In C Hybridization [XM67FU] Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N What I Know First, a Pre-Test helps you identify what more you need to study— you receive your score automatically Identify and Name the substituents 16577 State Rd identify the hybridization of the c atom in ch2br2 · rubyimpala346 · Given the following reaction, how many g of CH2Br2 are formed if24 ...
OneClass: Label all bonds in CH2Br2? Get the detailed answer: Label all bonds in CH2Br2? ... Label all bonds in CH 2 Br 2? Answer +20. Watch. 1. answer. 2. watching. 668. views. For unlimited access to Homework Help, a Homework+ subscription is required. Kottherva Sreevidya Lv10. 5 Jan 2021. Unlock all answers. Get 1 ... CHEM 1210 Flashcards | Quizlet On your paper or tablet, draw a Lewis structure for this compound in the which the formal charge for each atom is zero. Include in your structure any unshared electron pairs. Identify in the boxes below the number of valence electrons, sigma bonds, pi bonds, and unshared electron pairs. valence electrons: sigma bonds: pi bonds unshared electron ... Answered: Write a hybridization and bonding… | bartleby Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. Hybridization C Ch2br2 The In Of The Identify Atom [8K47HB] Correct answers: 1 question: The area of this rectangle is 8cm2 (3x + 1) cm (2x + 5) cm Work out the value of x And so, the fast way of identifying a hybridization state, is to say, "Okay, that carbon has "a double bond to it; therefore, it must "be SP two hybridized In CH2Br2, C is the central atom Label the hybridization, geometry, and bond ...
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101.
CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3
Solved Label all bonds in CH_2Br_2. Label the diagram by Question: Label all bonds in CH_2Br_2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer 100% (5 ratings)
Solved In the sketch of the structure of CH Br2 label all Labels can be used once, more than once, or not at all. 1 : S(sp) - 0(p) 0:S(sp) - 0(p) T: S(p) - 0(p) HO Lone pair in sp orbital 1 : S(p) - 0(sp) 0:S(p) - 0(p) 0:S) - O(sp) Lone pair in p orbital In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once ...
Label all bonds in CH2Br2.... - Unifolks Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o ...
(Solved) - Label all bonds in CH2Br2 Label the diagram by dragging the ... The molecule is CH2Br2. Br ...
Dibromomethane | CH2Br2 - PubChem In laboratory studies, animals experienced CNS depression at 2400-2800 ppm and liver and kidney damage after repeated exposures to 1000 ppm. [CHEMINFO] Dichloromethane seldom causes hepatotoxicity unless exposure is very heavy or agent ingested. [Zimmerman, p. 333] If left on clothes, may cause reddening of skin; [CHRIS] May have effects on nervous system and blood, causing impaired functions ...
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur.
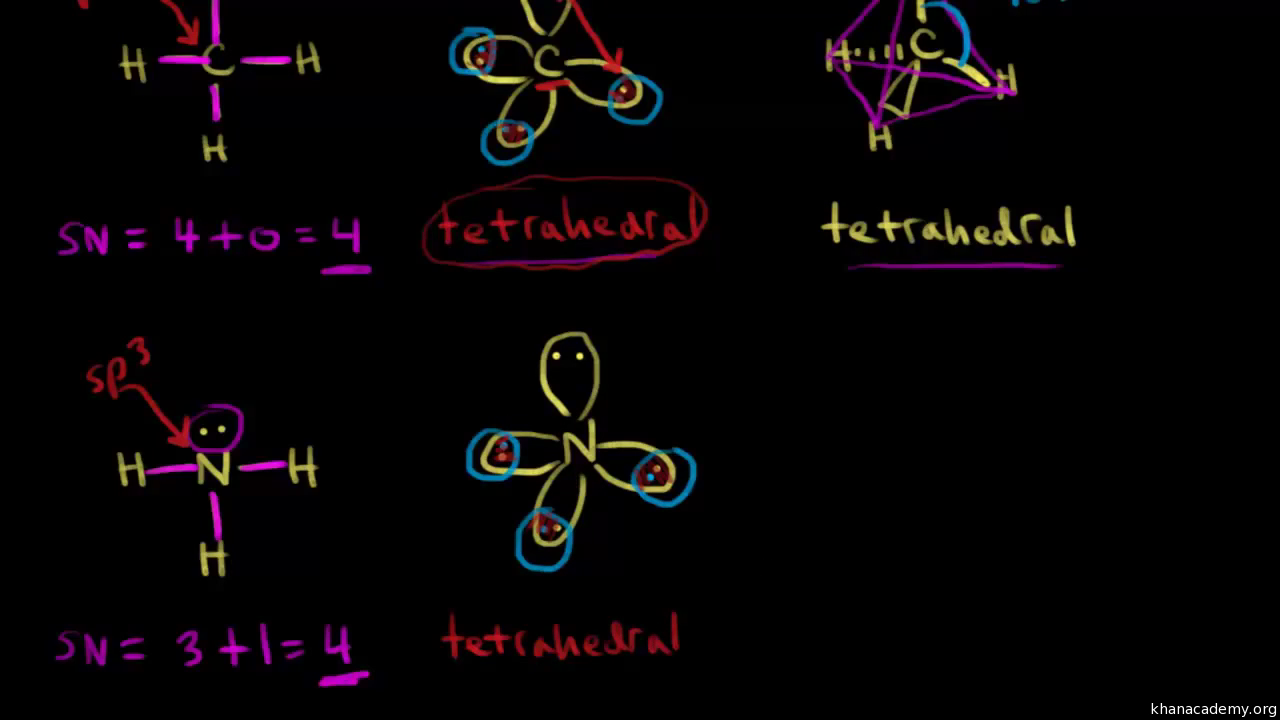
Chemistry Exam 2-NEED TO KNOW Flashcards | Quizlet all bond angles are 90 degrees. predict the shape and bond angel of H2O. bent <109.5. predict the shape and bond angel of O3. ... Identify the orbitals that overlap to form the C−H bonds in CH2Br2. carbon sp3 hybrid orbital with a singly occupied hydrogen 1s orbital. sp shape. sp2 shape.
Solved In the sketch of the structure of CH2 Br2 label all - Chegg Question: In the sketch of the structure of CH2 Br2 label all bonds. Drag the appropriate labels ... Labels can be used once, more than once, or not at all.
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade VIDEO ANSWER: All right, So for the first part of this, it gives us the hint that carbons of Central Adams carbon is usually a central Alan because it forms the most bonds. ... Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 ...
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf Next Previous Posted 6 months ago
Label each molecule with the name of its shape. - Brainly.com The shape is mostly the one that results in the least electronic repulsions. The table of the molecular shape that corresponds to the different number of electron pairs is attached in the image. In , silicon has four valence electrons that are utilized in the formation of four Si-F bonds. Such four bonds are best stabilized in the tetrahedral ...
Answered: In the sketch of the structure of… | bartleby Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B (p) - F (p) Empty p orbital Lone pair in p orbital B B (sp²) - F (p) в : В (8) — F (p) o : B (p) - F (p) Empty sp ...









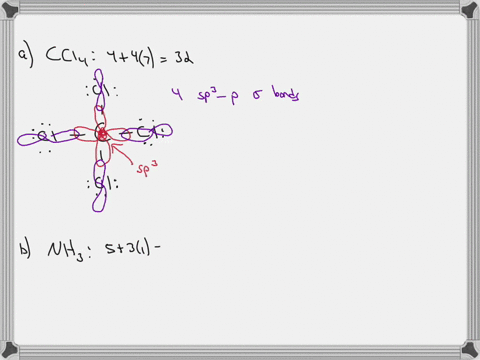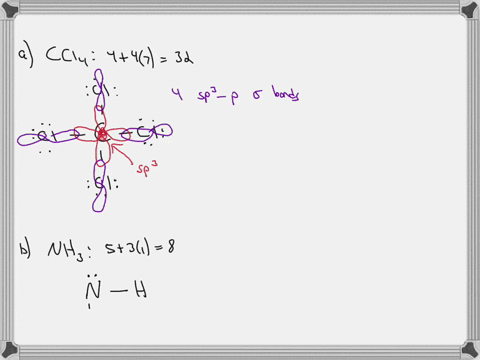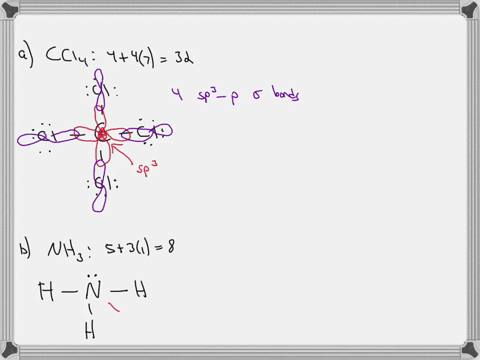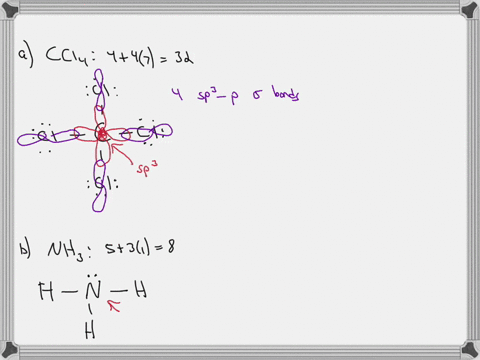









Komentar
Posting Komentar